In 2021, I had the fantastic opportunity to embark on a year-long Fulbright in South Korea to work on AI research under the supervision of a radiation oncologist. At first, I wasn’t exactly sure how this technology could directly add value in the lives of patients and physicians but with time, it became very clear to me, and I want to share what I learned with you.
Over my year, I began to piece together that these AI imaging models are important for three main reasons:
- It saves time (for both doctors and patients)
- It saves money (mainly for the hospital)
- It is accurate at drawing the boundaries between healthy organs vs cancer and at assisting with diagnoses
Now, let’s walk through an example of how a patient diagnosed with breast cancer –my supervisor specialized in breast cancer treatment so I choose this example but it can generalize to any cancer type– stands to benefit from AI imaging models. So, here is how the workflow goes:
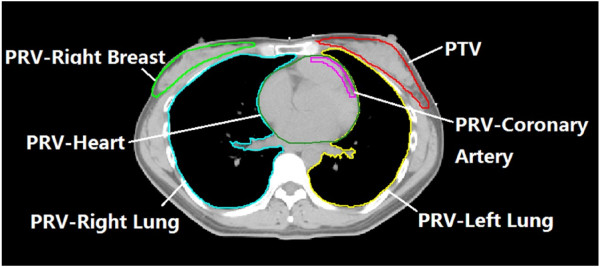
First, the patient needs to get imaging done, usually this is in the form of getting CT scans of the chest. These CT scans are really just a bunch of 2D cross sectional images that are put together to render a 3D image of a chest. These images are then uploaded to a computer where a radiation oncologist scrolls through each of the 2D images, drawing in the boundaries between healthy organs vs cancerous tissue (only someone with the medical training to identify these structures can do this –see the image above for an example). Once all these boundaries are drawn, you have a 3D image of the boundaries between all the healthy organs vs cancer in the patient. This process of drawing with an stylus on the computer screen can take up to an hour per patient depending on how many CT scans were taken (usually ~80) and the years of training that the physician has.
The next step is for radiation to be shot along the path of the boundaries drawn up to kill as much cancerous tissue as possible while preserving the health tissue. As a note, radiation therapy is only one type of treatment option for cancer with others being surgery and chemotherapy (these modules can also be combined).
Now, the process I described to you is the current work flow or standard of treatment for breast cancer at most hospitals. However, using machine learning, my supervisor began using previously contoured (i.e., drawn up) CT scans to train models to learn how to distinguish healthy organs from breast cancer tissue. Much of his previously published work along with the papers we worked on together showed that the performance or accuracy of contouring was largely comparable to an expert radiologist.
The implications of this technology are huge as the time per patient drawing contours could be reduced by up to 1 hour without sacrificing quality of treatment. The key with AI imaging models is really to make sure that they are performing at or above the capabilities of doctors manually doing the contouring or diagnosing. From there, it is pretty easy to see how how all parties –hospitals, physicians, patients– all stand to benefit with reduced burden of cost and time.
As a quick note, if you are a physician reading this, you may be thinking, ‘hey, well radiation oncologists do contouring, but radiologists –which is a completely separate specialization– are the ones actually do the diagnoses, labeling and reading the imaging studies. You are right to think this, but the imaging models are still beneficial and relevant to both fields. In rad onc, the contouring is automated. In diagnostic radiology, the labels or overall diagnoses are automatically predicted based on imaging models.
Now that I gave you a cursory view of the benefits and promise of this technology –assuming all goes right– I’d be remiss not to mention some of the challenges. Feel free to skip this part if you were just here for a high level overview. But, if you want a more granular understanding of the roadblocks, stay with me for a little longer.
In short, the major concerns with AI imaging model integration include data privacy and the need for extensive validation and standardization of AI tools in clinical settings. Furthermore, to go from the testing stage/world of academic studies, to the real-world applications in daily practice, it will take an interdisciplinary team of insights from healthcare professionals, technologists, and policymakers to implement this technology in a high-impact fashion.
I chose to keep this article succinct so I won’t go super deep into each of the aforementioned challenges, but I will link here a great review article that you can further dig into (although, I made sure to mention the major points throughout my article).
References:
“Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging.” Diagnostics 13, no. 2760 (2023). doi:10.3390/diagnostics130202760.
“Featured image source: Comparative dosimetric study for treating left-sided breast cancer in small breast sizes using various radiotherapy techniques. Source: Jin et al., Radiation Oncology (2013)”